Co2+ induced phase transformation from δ- to α-MnO2 and their hierarchical α-MnO2@δ-MnO2 nanostructures for efficient asymmetric supercapacitors†
Abstract
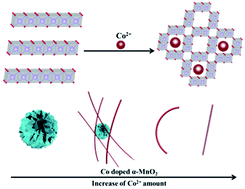
MnO2 possesses multiple crystal phases and various geometrical morphologies, so there remains a big challenge to simultaneously control the crystal phases and geometrical morphologies of MnO2 by a facile process. Indeed, these controllable syntheses will provide material bases to investigate their effects on the specific capacitance. Herein, benefiting from the first attempt of using Co2+ as a phase transformation-inducing agent and a doping ion, we present an interesting report on controllable phase transformation from δ- to α-MnO2 and their morphology evolution from nanosheets to nanowires. These transformations need the synergistic effect between MnCO3 and Co2+. Furthermore, three dimensional hierarchical α-MnO2@δ-MnO2 core–shell heterophase nanostructures were fabricated to improve capacitance performance. Impressively, the specific capacitance of α-MnO2@δ-MnO2 reaches up to 206 F g−1, which is higher than those of the corresponding single components. An asymmetric supercapacitor device based on the α-MnO2@δ-MnO2 cathode exhibits a good energy density of 12.9 W h kg−1 at a power density of 230 W kg−1 and excellent cycle lives with a decrease of 26% capacitance after 10 000 charge/discharge cycles.


 Please wait while we load your content...
Please wait while we load your content...