Influence of the gas atmosphere during the synthesis of g-C3N4 for enhanced photocatalytic H2 production from water on Au/g-C3N4 composites†
Abstract
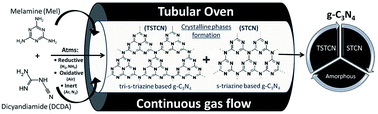
The design of Au/g-C3N4 nanocomposites for enhanced H2 production from water under solar and visible light irradiation is presented by varying the g-C3N4 synthesis atmosphere (air, N2, H2, Ar and NH3). We showed for the first time that the synthesis of g-C3N4 in a pure NH3 atmosphere led to enhanced photocatalytic performances between 3 and 9 times higher than g-C3N4 prepared in other gas atmospheres. The resulting, novel 0.3 wt% Au/g-C3N4–NH3 photocatalyst produced up to 324 μmol h−1 gcat−1 and 26 μmol h−1 gcat−1 of H2 corresponding to internal quantum yields of 1.85 and 0.60% under solar and visible light irradiation respectively, with an unusually low amount of triethanolamine used as the sacrificial agent (1 vol%). This enhanced activity was correlated to the structural, optical, porosity, and surface properties of g-C3N4, and to the quality of the interface with Au NPs. From an in-depth structure–activity correlation study, we highlighted the combined effects of a higher surface area with larger contribution of mesoporous volume, higher crystallization degree of g-C3N4–NH3 and lower deformation of nanosheets. Additionally, the ratio between tri-s-triazine and s-triazine based C3N4 was determined and used for the first time to point out the effect of different continuous gas flow atmospheres during synthesis. Furthermore, the suitable surface chemistry of g-C3N4–NH3 allowed more homogeneous coverage with small Au NPs yielding more intimate contact and higher quality of the interface between Au NPs and the g-C3N4 support.


 Please wait while we load your content...
Please wait while we load your content...