Promoting defective-Li2O2 formation via Na doping for Li–O2 batteries with low charge overpotentials†
Abstract
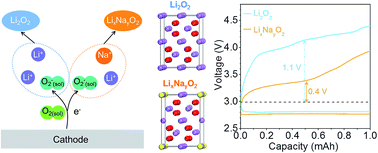
Li–O2 batteries represent a promising candidate for next-generation energy storage systems because of their high specific energy density. However, one key limitation is their low energy efficiency, which is caused by the high charge overpotential owing to the sluggish oxidation kinetics of the insulating Li2O2 product. Here, we report an approach to reduce charge overpotentials by forming Na-doped defective Li2O2 in a Na+-added electrolyte, which is verified by systematic experimental characterization. Theoretical simulations reveal that the Na-doped Li2O2 with lithium vacancies exhibits new conducting states right above the valence band edge, thereby enhancing electron transport properties. The cell with the defective-Li2O2 product achieves a charge overpotential of ∼0.4 V, much lower than that of the cell with Li2O2 (∼1.1 V). This study offers an effective approach to promote Li2O2 decomposition by forming defective Li2O2.


 Please wait while we load your content...
Please wait while we load your content...