Structural prediction and multilayer Li+ storage in two-dimensional VC2 carbide studied by first-principles calculations†
Abstract
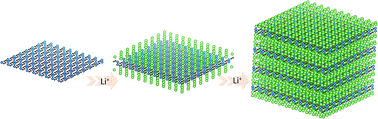
VC2, a new two-dimensional transition metal carbide containing C2 dimers, was predicted by the swarm-intelligent global-structure search method. The structural properties and Li+ storage ability of VC2 monolayers and stacked VC2 multilayers were systematically investigated by first-principles calculations, and the high structural stability and electronic conductivity of the materials suggested promising Li+ storage properties. VC2 monolayers showed a theoretical capacity of 1073 mA h g−1 based on multilayer Li+ adsorption, while stacked VC2 showed an even larger theoretical capacity of 1430 mA h g−1. Intercalated Li+ formed ordered arrangements between VC2 layers, retaining a well-ordered layered structure. Li+ near the VC2 layer formed ionic bonds with the host material, while Li in middle layers formed metallic Li–Li bonds. All Li+ was stored in the interlayer space with low diffusion barriers, which demonstrated high rate capability of the material for lithium ion batteries. Remarkably, the predicted VC2 carbide achieved more than 1000 mA h g−1 capacity irrespective of being in monolayer or stacked layer structures, which rendered them very convenient for practical material preparation and battery applications.


 Please wait while we load your content...
Please wait while we load your content...