Increase and discretization of the energy barrier for individual LiNixCoyMnyO2 (x + 2y =1) particles with the growth of a Li2CO3 surface film†
Abstract
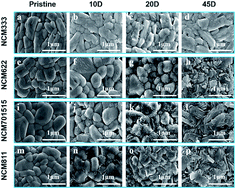
Surface degradation is a common challenge for many electrode materials. The active surface usually reacts with the molecules in the surrounding environment to form byproducts that hinder the diffusion channels for Li ions and electrons, increase the energy barrier for (de)lithiation reactions, and ultimately shorten the cycle life. Herein, the growth of surface Li2CO3 on LiNixCoyMnyO2 (x = 0.33, 0.6, 0.7, 0.8, x + 2y = 1) cathodes upon storage has been systematically investigated. Ni-rich surfaces are found to result in more Li2CO3 growth, based on which three discrete degradation models for layered oxides are proposed. The increase and discretization of the energy barrier for individual particles also explain the State-of-Charge heterogeneity phenomena observed by in situ XRD and the change of cyclic voltammetry curves. By providing a comprehensive picture of surface deterioration of the NCM cathode family, this study enhances the understanding of the degradation mechanism that determines the cycle life of electrode materials.


 Please wait while we load your content...
Please wait while we load your content...