Hierarchical tri-functional electrocatalysts derived from bimetallic–imidazolate framework for overall water splitting and rechargeable zinc–air batteries†
Abstract
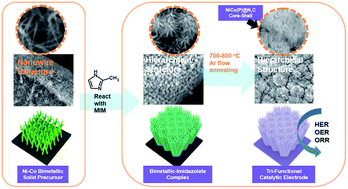
There is a growing need for an efficient multi-functional electrocatalyst that can produce industry-level high currents at low overpotentials. Herein, we report a highly efficient, robust tri-functional catalytic electrode that simultaneously catalyzes three different electrochemical reactions: the oxygen evolution reaction (OER), hydrogen evolution reaction (HER), and oxygen reduction reaction (ORR). The catalytic electrodes with a hierarchical structure are converted from a hierarchical nickel-rich, nickel–cobalt bimetallic–imidazolate framework directly grown onto a nickel foam. Catalytic electrodes composed of bimetallic phosphides exhibit excellent OER and HER catalytic activity (an overpotential of 201/250 mV at 20/100 mA cm−2 for OER and an overpotential of 67/110 mV at 20/100 mA cm−2 for HER), as well as ORR activity with a half-wave potential at 0.82 V vs. RHE. Furthermore, the bimetal–nitrogen–carbon (M–N–C) catalytic electrode also exhibits tunable tri-functional catalytic activity, including excellent ORR activity with a half-wave potential at 0.88 V vs. RHE. The high potential of these multi-functional electrocatalysts from single nickel-rich bimetallic–organic complexes is demonstrated by employing them as robust alkaline water electrolyzers, as well as decoupled air electrodes for rechargeable zinc–air batteries (ZABs).


 Please wait while we load your content...
Please wait while we load your content...