A Li2CuPS4 superionic conductor: a new sulfide-based solid-state electrolyte†
Abstract
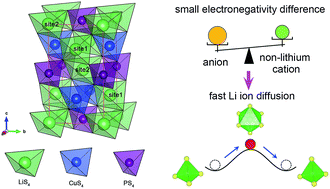
A new sulfide-based superionic conductor, a kesterite-structured Li2CuPS4 (LCPS) material, was proposed based on density functional theory (DFT) calculations. Our theoretical studies reveal that the LCPS material is thermodynamically and dynamically stable, and very likely to be experimentally synthesized. LCPS can form electronically insulating but ionically conducting interphases at high lithium chemical potential, preventing further reduction or oxidation by passivating its surface and enhancing its electrochemical stability due to the limited kinetics. LCPS is a superionic conductor, exhibiting a much higher ionic conductivity of 84.9 mS cm−1 at 300 K than the state-of-the-art Li10GeP2S12 material, due to the weaker Li ion binding and the energy compensation for forming Li vacancies due to the variable valence state of the Cu+ cation. The LCPS superionic conductor is promising for use as a solid-state electrolyte material for all-solid-state lithium ion batteries. Moreover, an alternative design principle for fabricating new superionic conductors with Li tetrahedral occupation by reducing the electronegativity difference between the anion element and non-lithium cation elements was identified for the first time.


 Please wait while we load your content...
Please wait while we load your content...