Oxygen vacancies promoting the electrocatalytic performance of CeO2 nanorods as cathode materials for Li–O2 batteries†
Abstract
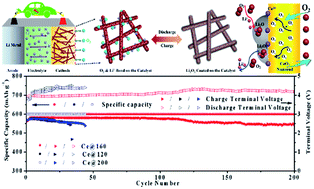
Li–O2 batteries have become very promising power sources for electronic vehicles as a result of their extraordinary energy density. Nevertheless, the unfavourable electrocatalytic activity of cathode materials in Li–O2 batteries is still a limiting factor for the practical application of Li–O2 batteries. This study proposes a surface engineering strategy which can enhance the electrocatalytic activity of CeO2 nanorods by tuning the oxygen vacancies on their surface, and found that the highest concentration of oxygen vacancies induces the best electrochemical performance, including an extended electrochemical stability of 200 cycles, and reduces the overpotential of the ORR from the reported 0.26 V to 0.11 V. Ex situ XPS photoelectron spectroscopy was carried out to further explain the role of oxygen vacancies in improving the electrochemical performance of LOBs, indicating that the oxygen vacancies of CeO2 nanorods have more obvious positive effects on the ORR than on the OER. It is believed that they can serve as the active sites for the deposition of Li2O2 films by being involved in the reaction between Li+ and O2 during the ORR, and also boost the electron transport through the insoluble Li2O2 films to further catalyse the Li+ and O2 reaction during the discharge and charge process. This work provides new proof for the association between the discharge/charge behaviour of LOBs and the content of oxygen vacancies.


 Please wait while we load your content...
Please wait while we load your content...