The efficient faradaic Li4Ti5O12@C electrode exceeds the membrane capacitive desalination performance†
Abstract
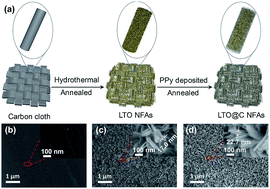
A faradaic reaction-enhanced membrane capacitive deionization (MCDI) system has recently been proposed for the desalination of seawater owing to its superior salt removal capacity, lower energy consumption and applicability in high-salinity water. However, the detailed mechanism of the effect of the faradaic reaction on the desalination performance of an MCDI system in the constant voltage (CV) and constant current (CC) operational modes has been rarely studied. Herein, we systematically studied the faradaic reaction in an MCDI system in the CV and CC operational modes based on carbon cloth, Li4Ti5O12 nanoflake array (LTO NFA), and carbon-coated Li4Ti5O12 nanoflake array (LTO@C NFA) electrodes; the XPS and ex situ XRD results verified the intercalation of sodium ions in different operational modes. Upon the introduction of the faradaic mechanism, the desalination capacity (DC) of the MCDI system was no longer limited by the surface area of the electrodes since the salt ions could intercalate into the bulk material in addition to being adsorbed by electrical double layers (EDLs). A high DC of 25 mg g−1 after over 30 cycles in the CC mode has been achieved, exceeding that of the carbon cloth without faradaic reactions by 300%. The unique combination of the CC operational mode and the faradic electrodes provides a new perspective for the design of high-efficiency electrodes for advanced CDI.


 Please wait while we load your content...
Please wait while we load your content...