High-performance graphene oxide nanofiltration membrane with continuous nanochannels prepared by the in situ oxidation of MXene†
Abstract
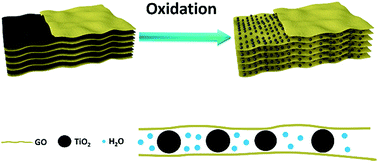
Separation membrane with laminar structure, assembled by two-dimensional materials, has been widely explored to achieve precise molecular separation and high water permeability in the field of water purification. Herein, a novel GO-based nanofiltration membrane intercalated with TiO2 nanoparticles was fabricated by the in situ oxidation of MXene nanosheets. The formation of TiO2 nanocrystals, derived from the oxidation of MXene, created additional channels for water permeation. Particularly, the consecutively distributed TiO2 nanocrystals acted as intercalators owing to their laminar structure and created continuous nanochannels within GO membrane for water transport, which shows great advantages over the membrane prepared using traditional methods. Water permeability of the obtained membrane reached 89.6 L m−2 h−1 bar−1, which is 7.3 times that of pristine GO membrane and 2.4 times that of GO/TiO2 membrane fabricated using traditional methods. The rejection rate for four different organic dyes is above 97%. The highly improved water permeability with no obvious sacrifice of rejection rate was attributed to the presence of continuous nanochannels obtained via the in situ oxidation of MXene without destroying the intrinsic stacking structure of GO sheets. This study offers a novel strategy to fabricate continuous nanochannels within the separation membrane with a two-dimensional laminar structure.


 Please wait while we load your content...
Please wait while we load your content...