Sodium iron sulfate alluaudite solid solution for Na-ion batteries: moving towards stoichiometric Na2Fe2(SO4)3†
Abstract
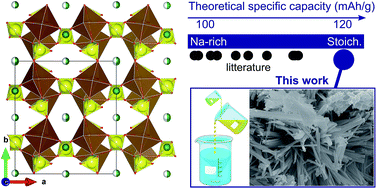
Thanks to the inductive effect of the sulfate groups, sodium iron sulfate alluaudites display the highest electrode potential amongst the Fe-based compounds studied in sodium-ion batteries. Here, we report the synthetic strategy that has allowed us to obtain the elusive Na2Fe2(SO4)3 stoichiometric compound through a reverse-strike coprecipitation method in organic medium. We experimentally confirm the hypothesis that the stoichiometric compound transforms upon further heat treatment into the previously reported sodium-rich solid solution and an iron sulfate secondary phase. X-ray diffraction and 57Fe Mössbauer spectroscopy do not reveal any striking structure difference between the stoichiometric and Na-rich compounds, in agreement with the current understanding that the instability of the stoichiometric phase is due to the repulsion between Fe2+ ions in the Fe2O10 dimers bridged by sulfate groups. Despite less-than-optimal powder microstructure, electrochemical activity of the stoichiometric phase could be demonstrated through operando X-ray diffraction. These findings are expected to shift attention towards the (near)-stoichiometric compositions, which offer the highest theoretical specific capacities thanks to their optimal Na/Fe ratio.


 Please wait while we load your content...
Please wait while we load your content...