Electrospray deposition-induced ambient phase transition in copper sulphide nanostructures†
Abstract
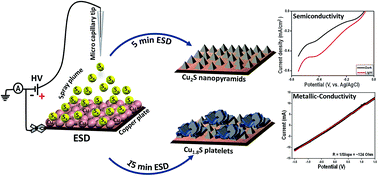
We introduce a new and simple method for synthesizing different phases of copper sulphide nanostructures using electrospray deposition (ESD) of molecular sulphur in the form of droplets on metallic copper surfaces under ambient conditions. Different phases of copper sulphide nanostructures were created by controlling the deposition time. Time dependent electron microscopy reveals conversion of the Cu2S nanopyramids to Cu1.8S platelets during the course of ESD. In the beginning of deposition, direct interaction between sulphur ions and metallic copper creates Cu2S nanopyramids followed by subsequent slow diffusion of sulphur leading to the formation of copper deficient Cu1.8S platelets. A detailed characterization of both the nanostructures was performed by using different microscopic and spectroscopic tools such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), Raman spectroscopy, powder X-ray diffraction (PXRD) and X-ray photoelectron spectroscopy (XPS). We have also studied the optical properties of these nanostructures in both UV-Vis and near infrared (NIR) regions. The characteristic broad peaks in the UV-Vis region of Cu2S nanopyramids indicate the photosensitive nature of the material. A positive photocurrent response was observed from the Cu2S nanopyramids under electrochemical conditions, while Cu1.8S nanostructure shows an intense localized surface plasmon (LSPR) peak in the NIR region indicating its metallic nature. Current–voltage (I–V) measurements showed metallic conductivity in them.


 Please wait while we load your content...
Please wait while we load your content...