Rechargeable Na/Ni batteries based on the Ni(OH)2/NiOOH redox couple with high energy density and good cycling performance†
Abstract
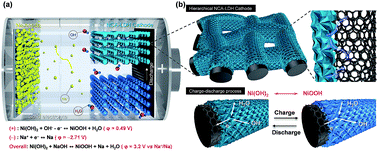
Rechargeable battery systems that use Na-based anodes as alternatives to Li-ion batteries are highly desirable for grid-scale energy storage systems owing to the high abundance and low cost of Na. Furthermore, aqueous Na batteries are advantageous considering the cost, safety and cycle life. However, the limited energy density is still a critical issue for Na-based batteries. Here, we demonstrate a high performance rechargeable battery using dual electrolytes based on a Na metal anode and a redox couple of hierarchical NiCoAl-layered double hydroxide (NiCoAl-LDH) nanosheets on a carbon microfiber electrode with high energy storage capacity. In this design, the wide potential range of the Na metal anode and the high capacity of hierarchical NiCoAl-LDH nanosheets on a carbon microfiber cathode enable a rechargeable Na/Ni battery with excellent energy storage performance. For stable operation in a hybrid system using non-aqueous and aqueous electrolytes, an alkali-ion solid electrolyte (NASICON, Na3Zr2Si2PO12) is used for the separation of electrolytes. The Na/Ni battery exhibits a stable operating voltage of ∼3.1 V during discharge which outperforms the low cell voltage (∼1.23 V) of an aqueous rechargeable battery, a high capacity of ∼350 mA h g−1, and a resulting high energy density of ∼1085 W h kg−1. With the combination of a solid-state redox couple as the cathode and a metallic sodium anode, our study demonstrates the high potential of Na based batteries for high energy EES systems.


 Please wait while we load your content...
Please wait while we load your content...