A boron-interstitial doped C2N layer as a metal-free electrocatalyst for N2 fixation: a computational study†
Abstract
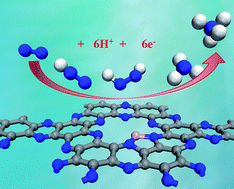
Electrochemical reduction of the naturally abundant nitrogen (N2) in aqueous solutions under ambient conditions is a quite promising way for ammonia (NH3) synthesis, in which the development of highly efficient electrocatalysts is a key scientific issue. Herein, by means of extensive density functional theory (DFT) computations, we proposed that a boron-interstitial (Bint)-doped C2N layer can act as a metal-free electrocatalyst for N2 fixation and reduction to NH3. Our computations revealed that the Bint-doped C2N layer can sufficiently activate the N2 molecule through the “acceptance-donation” process due to its significant positive charge and magnetic moment on the B dopant. In particular, the subsequent N2 reduction reaction prefers to proceed via the enzymatic mechanism with a rather low limiting potential (−0.15 V), suggesting its superior catalytic performance for N2 reduction. Importantly, the Bint-doped C2N layer possesses comparable stability to the experimentally available S-doped one and thus holds great promise for experimental synthesis. Thus, by carefully controlling the doping sites, the B-doped C2N layer can be utilized as a quite promising metal-free catalyst with high-efficiency for N2 reduction, which may provide useful guidance for further facilitating sustainable NH3 synthesis.


 Please wait while we load your content...
Please wait while we load your content...