A general synthetic methodology to access magnesium aluminate electrolyte systems for Mg batteries†
Abstract
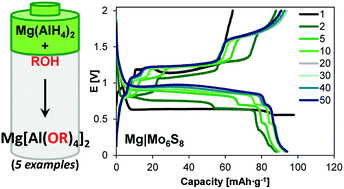
Magnesium electrolyte systems that are oxidatively stable, stable towards reduction at Mg plating potentials, and can be cycled for long periods without forming insulating surface films are crucial for the development of viable Mg batteries. Here, we report a series of Mg aluminate salts [Mg(AlOR4)2], prepared using a general synthetic approach that employs Mg(AlH4)2 and various alcohols, for use in electrolyte systems for Mg batteries. This methodology provides access to Mg aluminates that do not possess β-hydrogens and thereby preclude possible β-hydride elimination decomposition pathways, which have been reported for analogous aluminium-based electrolytes. The cycling of these systems using Au and Mg electrodes is strongly influenced by the presence of chloride, which is found to lower plating/stripping overpotentials and increase Coulombic efficiencies. Further, these Mg aluminates can be cycled efficiently in Mg full cells containing a Chevrel phase Mo6S8 cathode with good capacity retention.


 Please wait while we load your content...
Please wait while we load your content...