Atomic Fe hetero-layered coordination between g-C3N4 and graphene nanomeshes enhances the ORR electrocatalytic performance of zinc–air batteries†
Abstract
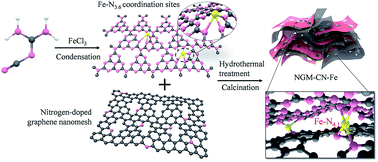
Hetero-layered iron–nitrogen coordination between g-C3N4 and graphene nanomeshes was developed for superior electrocatalytic activity in the oxygen reduction reaction. Compared with the performance of g-C3N4 or atomic Fe embedded in g-C3N4 in the oxygen reduction reaction, the current density at −0.5 V of the two-dimensional hetero-hybrid of atomic Fe, g-C3N4 and graphene was enhanced 13 times, and the half-wave potential of the hybrid positively shifted to 0.278 V. The hybrid exhibited superior electrocatalytic activity with a 20 mV more positive half-wave potential, higher current density, better methanol tolerance and longer-term stability compared to commercial Pt–C. This enhancement originated from mesh-on-mesh exposed inter-layer bridged Fe–N4.1 coordination active sites between g-C3N4 and graphene, which favored a four-electron pathway accompanied by the improvement of the conductivity and mass transport. Superior performance, including a low charge–discharge voltage gap over 20 h of cyling, of the hybrid-based Zn–air battery was achieved. This strategy of the hetero-layered interfacial metal–nitrogen coordination between different 2D materials is a general approach to develop advanced electrocatalysts for sustainable energy applications.


 Please wait while we load your content...
Please wait while we load your content...