Hydrogelation with a water-insoluble organogelator – surfactant mediated gelation (SMG)†
Abstract
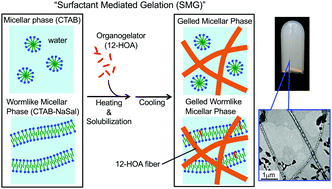
The low-molecular-weight gelator (LMG) 12-hydroxyoctadecanoic acid (12-HOA) is insoluble in water, but can be solubilized in surfactant micelles. We therefore solubilized 12-HOA at 80 °C in an aqueous solution of cetyltrimethylammonium bromide (CTAB) containing spherical micelles. On cooling this system down to room temperature, a hydrogel is obtained. We will refer to this process as “surfactant-mediated gelation” (SMG). The hydrogels were formed at a lower 12-HOA concentration when sodium salicylate (NaSal) was added to the CTAB system, which induced the formation of wormlike micelles. Hydrogels obtained by SMG from spherical and wormlike micelles are referred to as gelled micellar phases (GMs) and gelled wormlike micellar phases (GWLMs), respectively. Optical microscopy and transmission electron microscopy (TEM) showed that 12-HOA forms self-assembled fibrillar networks (SAFiNs) in both GMs and GWLMs. The sol–gel transition temperature, Tsol–gel, of the GWLM samples was higher than that of the GM samples. Dynamic rheological measurements revealed gel properties (G′ > G′′ at all angular frequencies) for both gels; however, a higher viscoelasticity was observed for the GWLM samples, which in turn, was reflected in the higher Tsol–gel. Small- and wide-angle X-ray scattering (SWAXS) showed that micelles and gel fibers coexist in the GM and GWLM samples. Our study demonstrates the gelation of aqueous micellar solutions with water-insoluble LMGs.


 Please wait while we load your content...
Please wait while we load your content...