Gelled non-toxic microemulsions: phase behavior & rheology
Abstract
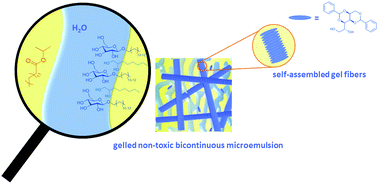
Bicontinuous microemulsions gelled with a low molecular weight gelator have been shown to be an orthogonally self-assembled system. With the mechanical stability provided by the gel network, gelled non-toxic bicontinuous microemulsions have the potential to be an efficient transdermal drug delivery carrier. However, up to now no suitable system has been formulated for transdermal drug delivery. To fill this gap, we formulated and characterized a gelled non-toxic bicontinuous microemulsion suitable for the mentioned application. Starting from a previously studied scouting system, namely, H2O–n-octane–n-octyl β-D-glucopyranoside (β–C8G1)–1-octanol, the co-surfactant and the oil were replaced by non-toxic components. Subsequently, the expensive pure surfactant was replaced by cheap technical-grade surfactants (Plantacare® series) to make the system economical. Having formulated the non-toxic microemulsion H2O–IPM–Plantacare 1200 UP–1,2-octanediol, three low molecular weight gelators were studied with regard to the gelation of both the scouting system and the non-toxic system. The chosen gelators were 12-hydroxyoctadecanoic acid (12-HOA), 1,3:2,4-dibenzylidene-D-sorbitol (DBS), and N,N′-dibenzoyl-L-cystine (DBC). We found that only DBS gels the non-toxic microemulsion. The gelled non-toxic bicontinuous microemulsion H2O–IPM–Plantacare 1200 UP–1,2-octanediol was characterized with oscillatory shear rheometry and small-angle neutron scattering (SANS) at a DBS concentration of 0.3 wt% to verify that the system is indeed a gel and that the microstructure of the microemulsion is not altered by the gel network.


 Please wait while we load your content...
Please wait while we load your content...