Natural rubber–SiO2 nanohybrids: interface structures and dynamics†
Abstract
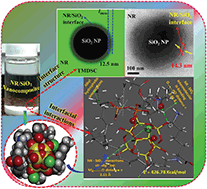
Homogeneous dispersion of silica nanoparticles (SiO2 NPs) in natural rubber (NR) is a key challenge for engineering high-performance nanocomposites and elucidation of their structure on a molecular basis. Towards this, the present work devised a novel route for obtaining 3D self-assembled SiO2 NP–NR nanocomposites under aqueous conditions and in the presence of Mg2+, by establishing a molecular bridge that clamped the negatively charged NR and SiO2 colloidal particles with a favoured NR–SiO2 NP hetero-aggregation. The characteristic NR–SiO2 NP hetero-aggregates displayed a decreased heat capacity with increase in the SiO2 mass-fraction, implying a restricted NR chain mobility. Such changes in the interfacial layers were tapped by 29Si NMR, DFT calculations and molecular dynamics simulations towards a mechanistic understanding of the structure and dynamics of the NR/SiO2 NP hybrid. Simple models were used to illustrate basic ideas; specific electrostatic interactions such as ion–dipole and H-bonding interactions proved to be the driving forces for the organized assembly leading to the NR–SiO2 hetero-aggregate over the NR–NR or SiO2 NP–SiO2 NP homo-aggregate. Molecular dynamics simulation of the aqueous canonical ensemble of the hybrid showed the stable molecular conformation to reveal a SiO2 NP spherical core encapsulated by a hydrophobically interconnected NR polymer layer as the outer shell, as a unique structural model. Specifically, the lipid end of the NR was involved electrostatically while the lysine end (the protein part of NR) H-bonded to the core silica cluster thereby restricting random aggregation. The calculated negative free energy changes for the hetero-aggregate composites via their vibrational and rotational spectra proved the spontaneity of composite formation.


 Please wait while we load your content...
Please wait while we load your content...