Controllable assembly of a novel cationic gemini surfactant containing a naphthalene and amide spacer with β-cyclodextrin†
Abstract
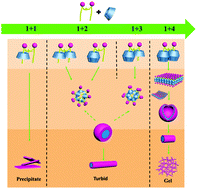
A novel cationic gemini surfactant (C12NDDA) with a spacer containing naphthalene and amides was successfully synthesized. The assembly of C12NDDA with β-cyclodextrin (β-CD) was investigated using various techniques including transmission electron microscopy, proton nuclear magnetic resonance (1H NMR), and scanning electron microscopy. Tuning the C12NDDA concentration and the C12NDDA/β-CD molar ratio allowed the production of different assembled aggregate morphologies such as micelles, vesicles, nanowires, nanorods, and hydrogels. Investigation of the inclusion mechanisms of C12NDDA and β-CD by 1H NMR revealed that hydrophobic interactions, hydrogen bonding, π–π stacking, and electrostatic forces play key roles in the assembly process. The antimicrobial activities of the C12NDDA/xβ-CD (x = 0–4) inclusion complexes were tested against Gram-negative bacteria (Escherichia coli and Salmonella) and Gram-positive bacteria (Staphylococcus aureus and Streptococcus), and very low minimum inhibitory concentrations of 0.078–0.31 μg mL−1 were observed. Thus, this newly synthesized gemini surfactant and its inclusion complexes exhibit potential as superior broad-spectrum disinfectants for various biomedical and biotechnological applications.


 Please wait while we load your content...
Please wait while we load your content...