Amorphous nanoparticles by self-assembly: processing for controlled release of hydrophobic molecules†
Abstract
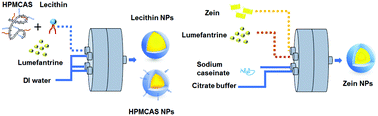
More than 40% of newly developed drug molecules are highly hydrophobic and, thus, suffer from low bioavailability. Kinetically trapping the drug as a nanoparticle in an amorphous state enhances solubility. However, enhanced solubility can be compromised by subsequent recrystallization from the amorphous state during drying processes. We combine Flash NanoPrecipitation (FNP) to generate nanoparticles with spray-drying to produce stable solid powders. We demonstrate that the continuous nanofabrication platform for nanoparticle synthesis and recovery does not compromise the dissolution kinetics of the drug. Lumefantrine, an anti-malaria drug, is highly hydrophobic with low bioavailability. Increasing the bioavailability of lumefantrine has the potential to reduce the dose and number of required administrations per treatment, thus reducing cost and increasing patient compliance. The low melting temperature of lumefantrine (Tm = 130 °C) makes the drying of amorphous nanoparticles at elevated temperatures potentially problematic. Via FNP, we produced 200–400 nm nanoparticles using hydroxypropyl methylcellulose acetate succinate (HPMCAS), lecithin phospholipid, and zein protein stabilizers. Zein nanoparticles were spray-dried at 100 °C and 120 °C to study the effect of the drying temperature. For zein powders, at two hours the dissolution kinetics under fasted conditions reached 85% release for the 100 °C sample, but only 60% release for the 120 °C sample. Powder X-ray diffraction, differential scanning calorimetry, and solid state nuclear magnetic resonance indicate that the lumefantrine in the nanoparticle core is amorphous for samples spray-dried at 100 °C. Dissolution under fed state conditions showed similar release kinetics for both temperatures, with 90–95% release at two hours. Zein and HPMCAS nanoparticles spray-dried at 100 °C showed release profiles in fasted and fed state media that are identical to those of lyophilized samples, i.e. those dried at cryogenic conditions where no transformation to the crystalline state can occur. Thus, spray drying 30 °C below the melting transition of lumefantrine is sufficient to maintain the amorphous state. These inexpensive formulations have potential to be developed into future therapies for malaria, and the results also highlight the potential of combining FNP and spray-drying as a versatile platform to assemble and rapidly recover amorphous nanoparticles in a solid dosage form.


 Please wait while we load your content...
Please wait while we load your content...