Protecting effect of mass transport during electrochemical reduction of oxygenated carbon dioxide feedstocks†
Abstract
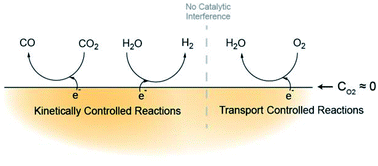
Electrochemical CO2 reduction is a promising path toward mitigating carbon emissions while also monetizing waste gas through chemicals production and storage of surplus renewable energy. However, deploying such a technology for use on industrial CO2 sources requires an understanding of the effects that gas feed impurities have upon CO2 reduction reaction (CO2RR). In this work, we elucidate the impact of molecular oxygen on the network of reactions occurring in a CO2 reduction system. Our findings indicate that for a planar, polycrystalline Au electrode in an aqueous environment, oxygen reduction current is limited by the transport characteristics specific to the cell geometry and solvent; as a result, mass transport confers a protective effect by mitigating the otherwise thermodynamically and kinetically favorable reduction of oxygen. The presence of oxygen does not appear to have a significant impact on either CO2RR or hydrogen evolution partial currents, indicating that the mechanisms of reduction reactions involving oxygen are independent of CO2RR and hydrogen evolution. Further, an electrokinetic mechanistic analysis indicates many feasible candidates for the rate-determining step of CO2RR; there is no indication that the CO2RR mechanism at PCO2 = 0.5 atm is altered by the presence of oxygen, as the Tafel slopes (59 mV dec−1) and reaction orders with respect to bicarbonate (0), CO2 (∼1.5), and protons (0 from lack of KIE) are consistent between systems with PO2 = 0 atm and those with PO2 = 0.5 atm. While this is promising for the robustness of CO2RR to oxygen impurities in gas feeds, the ultimate design tradeoff when utilizing CO2 sources containing oxygen is between the cost of separation processes and the corresponding cost of power inefficiency as a result of electrons lost to oxygen reduction. This represents a first step in understanding kinetic and transport considerations in the design of gas-impurity-tolerant CO2 reduction systems.


 Please wait while we load your content...
Please wait while we load your content...