Acoustic cavitation generates molecular mercury(ii) hydroxide, Hg(OH)2, from biphasic water/mercury mixtures†
Abstract
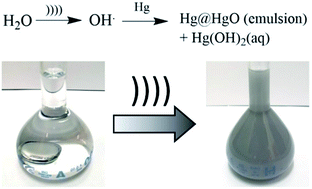
Emulsification of elemental mercury in aqueous solution in the form of grey particles occurs upon exposure to intense sound fields. We show the concomitant formation of molecular Hg(OH)2 in the solution phase reaching a saturation limit of 0.24 mM at 25 °C. The formation of Hg(OH)2 is consistent with the ‘hot spot’ model which suggests the formation of OH˙ as a result of acoustic cavitation; such radicals are proposed to combine with Hg to form the Hg(OH)2 species here characterised using voltammetry.


 Please wait while we load your content...
Please wait while we load your content...