Rh(ii)-catalyzed branch-selective C–H alkylation of aryl sulfonamides with vinylsilanes†
Abstract
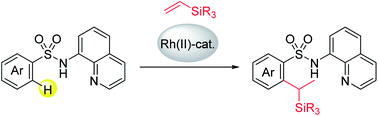
Rhodium(II)-catalyzed unusual branch-selective ortho-C–H alkylation of aryl sulfonamides with vinylsilanes was achieved using an 8-aminoquinoline directing group. Notably, the para-substituted aryl sulfonamides gave mono-(branched)alkylated products exclusively without the formation of any double C–H alkylated byproducts. The results of deuterium labeling experiments suggest that both hydrometalation and carbometalation pathways are involved in this conversion.


 Please wait while we load your content...
Please wait while we load your content...