Valency engineering of monomeric enzymes for self-assembling biocatalytic hydrogels†
Abstract
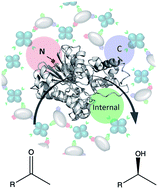
All-enzyme hydrogels are efficient reagents for continuous flow biocatalysis. These materials can be obtained by self-assembly of two oligomeric enzymes, modified with the complementary SpyTag and SpyCatcher units. To facilitate access to the large proportion of biocatalytically relevant monomeric enzymes, we demonstrate that the tagging valency of the monomeric (S)-stereoselective ketoreductase Gre2p from Saccharomyces cerevisiae can be designed to assemble stable, active hydrogels with the cofactor-regenerating glucose 1-dehydrogenase GDH from Bacillus subtilis. Mounted in microfluidic reactors, these gels revealed high conversion rates and stereoselectivity in the reduction of prochiral methylketones under continuous flow for more than 8 days. The sequential use as well as parallelization by ‘numbering up’ of the flow reactor modules demonstrate that this approach is suitable for syntheses on the semipreparative scale.


 Please wait while we load your content...
Please wait while we load your content...