Switching the site-selectivity of C–H activation in aryl sulfonamides containing strongly coordinating N-heterocycles†
Abstract
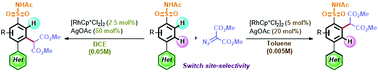
The limitations of arene C–H functionalization of aryl sulfonamides containing strongly coordinating N-heterocycles were overcome using a Rh(III) catalyst. The site-selectivity of C–H carbenoid functionalization at the ortho position relative to either the sulfonamide or N-heterocycle directing groups was elegantly switched using solvents of different polarities and different additive concentrations. Importantly, sulfonamide-group-directed ortho-C–H carbenoid functionalization tolerated strongly coordinating N-heterocycles, including pyridine, pyrrole, thiazole, pyrimidine, and pyrazine. Density functional theory (DFT) calculations were performed to rationalize the reaction mechanisms and the influence of reaction polarity.


 Please wait while we load your content...
Please wait while we load your content...