Synthesis of γ-substituted carbonyl compounds from DMSO-mediated oxidation of enynamides: mechanistic insights and carbon- and hetero-functionalizations†
Abstract
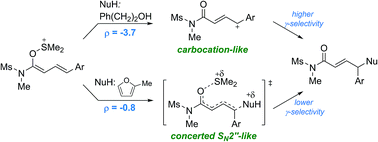
Oxidative coupling of 1,3-enynamides using DMSO as a terminal oxidant has been developed. Carbon as well as unmodified heteroatom nucleophiles, including aliphatic alcohols, thiols, and hydrazides, could be efficiently alkylated at the γ-position in a highly regioselective fashion. The kinetic analysis suggested a nucleophile-dependent mechanism ranging from a concerted SN2′′ to a carbocationic mechanism. Thus, the remote site-selectivity was ascribed to the partial positive charge developing at the terminal carbocationic center.


 Please wait while we load your content...
Please wait while we load your content...