Deoxygenative trifluoromethylthiolation of carboxylic acids†
Abstract
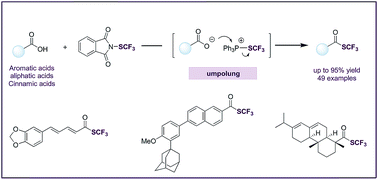
Here we describe a deoxygenative trifluoromethylthiolation method that yields trifluoromethyl thioesters from readily available carboxylic acids. The method is built upon an “umpolung” strategy where triphenylphosphine is used to first activate an electrophilic trifluoromethylthiolating reagent and then serves as an oxygen acceptor for the deoxygenation. The method is mild, efficient, broad-scope, and tolerant. It can be applied for the late-stage functionalization of numerous natural products and drug molecules containing a carboxylic acid group. The trifluoromethyl thioesters can be converted into trifluoromethyl thioethers by Pd-catalyzed decarbonylation.


 Please wait while we load your content...
Please wait while we load your content...