Molybdenum and tungsten complexes with carbon dioxide and ethylene ligands†
Abstract
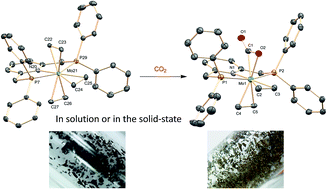
The first examples of stable metal complexes with coordinated ethylene and carbon dioxide ligands are reported. Reaction of tris(ethylene) complexes mer-M(C2H4)3(PNP) (M = Mo and W; PNP = 2,6-bis(diphenylphosphinomethyl)pyridine) with CO2 yields the corresponding, mixed cis-M(C2H4)2(CO2)(PNP) derivatives. X-ray studies reveal six-coordinate structures exhibiting η2-ethylene and κ2-C,O carbon dioxide coordination. Remarkably, the formation of the molybdenum CO2 adduct occurs also in the solid state at room temperature, under 4 bar of CO2, in a nearly quantitative manner.


 Please wait while we load your content...
Please wait while we load your content...