Palladium-catalyzed decarbonylative Suzuki–Miyaura cross-coupling of amides by carbon–nitrogen bond activation†
Abstract
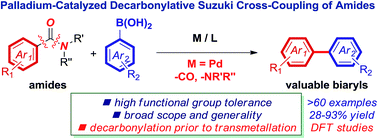
Palladium-catalyzed Suzuki–Miyaura cross-coupling or aryl halides is widely employed in the synthesis of many important molecules in synthetic chemistry, including pharmaceuticals, polymers and functional materials. Herein, we disclose the first palladium-catalyzed decarbonylative Suzuki–Miyaura cross-coupling of amides for the synthesis of biaryls through the selective activation of the N–C(O) bond of amides. This new method relies on the precise sequence engineering of the catalytic cycle, wherein decarbonylation occurs prior to the transmetallation step. The reaction is compatible with a wide range of boronic acids and amides, providing valuable biaryls in high yields (>60 examples). DFT studies support a mechanism involving oxidative addition, decarbonylation and transmetallation and provide insight into high N–C(O) bond activation selectivity. Most crucially, the reaction establishes the use of palladium catalysis in the biaryl Suzuki–Miyaura cross-coupling of the amide bond and should enable the design of a wide variety of cross-coupling methods in which palladium rivals the traditional biaryl synthesis from aryl halides and pseudohalides.


 Please wait while we load your content...
Please wait while we load your content...