Alkynylation of radicals: spotlight on the “Third Way” to transfer triple bonds
Abstract
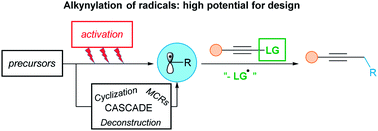
The alkynylation of radical intermediates has been known since a long time, but had not been broadly applied in synthetic chemistry, in contrast to the alkynylation of either electrophiles or nucleophiles. In the last decade however, it has been intensively investigated leading to new disconnections to introduce versatile triple bonds into organic compounds. Nowadays, such processes are important alternatives to classical nucleophilic and electrophilic alkynylations. Efficient alkyne transfer reagents, in particular arylsulfones and hypervalent iodine reagents were introduced. Direct alkynylation, as well as cascade reactions, were subsequently developed. If relatively harsh conditions were required in the past, a new era began with progress in photoredox and transition metal catalysis. Starting from various radical precursors, alkynylations under very mild reaction conditions were rapidly discovered. This review covers the evolution of radical alkynylation, from its emergence to its current intensive stage of development. It will focus in particular on improvements for the generation of radicals and on the extension of the scope of radical precursors and alkyne sources.


 Please wait while we load your content...
Please wait while we load your content...