α-Silicon effect assisted Curtin–Hammett allylation using allylcopper reagents derived from 1,3-dienylsilanes†
Abstract
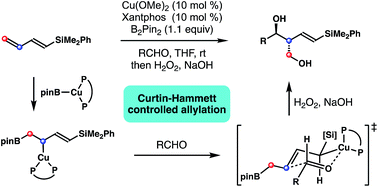
Cu-catalyzed stereoselective synthesis of (E)-δ-silyl-anti-homoallylic alcohols from 1,3-dienylsilane was developed. Mechanistic studies revealed that the borocupration of dienylsilane proceeded through a 1,2-addition pathway to give an allylcopper intermediate with Cu distal to the silyl group. However, the subsequent aldehyde allylation proceeded via Curtin–Hammett control to give (E)-δ-silyl-anti-homoallylic alcohols with high diastereoselectivities. This method was applied to the synthesis of the C1–9 fragment of a polyketide natural product, mycinolide IV.


 Please wait while we load your content...
Please wait while we load your content...