Frontiers and progress in cation-uptake and exchange chemistry of polyoxometalate-based compounds
Abstract
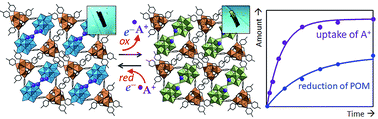
Cation-uptake and exchange has been an important topic in both basic and applied chemistry relevant to life and materials science. For example, living cells contain appreciable amounts of Na+ and K+, and their concentrations are regulated by the sodium–potassium pump. Solid-state cation-exchangers such as clays and zeolites both natural and synthetic have been used widely in water softening and purification, separation of metal ions and biomolecules, etc. Polyoxometalates (POMs) are robust, discrete, and structurally well-defined metal-oxide cluster anions, and have stimulated research in broad fields of sciences. In this perspective, cation-uptake and exchange in POM and POM-based compounds are categorized and reviewed in three groups: (i) POMs as inorganic crown ethers and cryptands, (ii) POM-based ionic solids as cation-exchangers, and (iii) reduction-induced cation-uptake in POM-based ionic solids, which is based on a feature of POMs that they are redox-active and multi-electron transfer occurs reversibly in multiple steps. This method can be utilized to synthesize mixed-valence metal clusters in metal ion-exchanged POM-based ionic solids.


 Please wait while we load your content...
Please wait while we load your content...