Computational design of syntheses leading to compound libraries or isotopically labelled targets†
Abstract
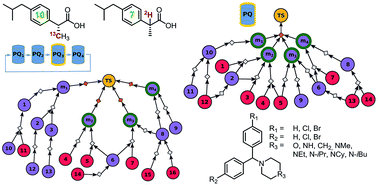
Although computer programs for retrosynthetic planning have shown improved and in some cases quite satisfactory performance in designing routes leading to specific, individual targets, no algorithms capable of planning syntheses of entire target libraries – important in modern drug discovery – have yet been reported. This study describes how network-search routines underlying existing retrosynthetic programs can be adapted and extended to multi-target design operating on one common search graph, benefitting from the use of common intermediates and reducing the overall synthetic cost. Implementation in the Chematica platform illustrates the usefulness of such algorithms in the syntheses of either (i) all members of a user-defined library, or (ii) the most synthetically accessible members of this library. In the latter case, algorithms are also readily adapted to the identification of the most facile syntheses of isotopically labelled targets. These examples are industrially relevant in the context of hit-to-lead optimization and syntheses of isotopomers of various bioactive molecules.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...