Tuning the π-bridge of quadrupolar triarylborane chromophores for one- and two-photon excited fluorescence imaging of lysosomes in live cells†‡
Abstract
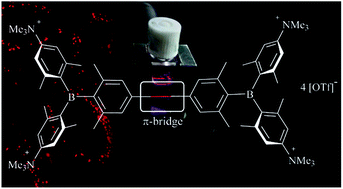
A series of tetracationic quadrupolar chromophores containing three-coordinate boron π-acceptors linked by different π-bridges, namely 4,4′-biphenyl, 2,7-pyrene, 2,7-fluorene, 3,6-carbazole and 5,5′-di(thien-2-yl)-3,6-diketopyrrolopyrrole, were synthesized. While their neutral precursors 1–5 displayed highly solvatochromic fluorescence, the water-soluble tetracationic target molecules 1M–5M, did not, but their emission colour could be tuned from blue to pink by changing the π-bridge. Compound 5M, containing the diketopyrrolopyrrole bridge, exhibits the most red-shifted absorption and emission maxima and the largest two-photon absorption cross-section (4560 GM at 740 nm in MeCN). Confocal laser scanning fluorescence microscopy studies in live cells confirm localization of the dye at the lysosome. Moreover, the low cytotoxicity, and high photostability of 5M combined with two-photon excited fluorescence imaging studies demonstrate its excellent potential for lysosomal imaging in live cells.
- This article is part of the themed collections: Most popular 2019-2020 inorganic, main group and crystal engineering chemistry articles and Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...