One metal is enough: a nickel complex reduces nitrate anions to nitrogen gas†
Abstract
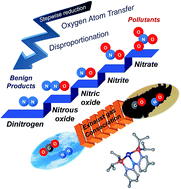
A stepwise reduction sequence from nitrate to dinitrogen gas at a single nickel center was discovered. A PNP nickel scaffold (PNP− = N[2-PiPr2-4-Me-C6H3]2) emerged as a universal platform for the deoxygenation of NOx substrates. In these reactions carbon monoxide acts as the oxygen acceptor and forms CO2 to provide the necessary chemical driving force. Whereas the first two oxygens are removed from the Ni-nitrate and Ni-nitrite complexes with CO, the deoxygenation of NO requires a disproportionation reaction with another NO molecule to form NO2 and N2O. The final deoxygenation of nitrous oxide is accomplished by the Ni–NO complex and generates N2 and Ni–NO2 in a relatively slow, but clean reaction. This sequence of reactions is the first example of the complete denitrification of nitrate at a single metal-site and suggests a new paradigm of connecting CO and NOx as an effective reaction pair for NOx removal.
- This article is part of the themed collections: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS), 2019 Chemical Science HOT Article Collection and Editor's Choice – Serena DeBeer


 Please wait while we load your content...
Please wait while we load your content...