Sequence isomerism-dependent self-assembly of glycopeptide mimetics with switchable antibiofilm properties†
Abstract
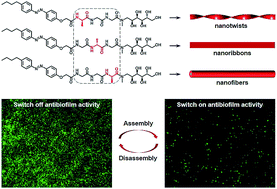
In biological systems, diverse amino acid sequences and functional decorations endow proteins with specific functions. Functionally modified oligopeptides are attractive building blocks to assemble stimuli-responsive biomimetic superstructures for mimicking soft structures in nature and biomaterial applications. In this work, we selectively synthesized the structurally simplest isomeric tripeptides (i.e., Ala–Gly–Gly–OH, Gly–Ala–Gly–OH and Gly–Gly–Ala–OH) to demonstrate how the subtlest change in sequence isomerism influences the self-assembly of glycopeptides. To impart self-assembly capability and stimuli-responsiveness, the isomeric tripeptides were modified with a hydrophobic n-butylazobenzene tail at the N-terminal. We observed three different self-assembled 1-D morphologies (i.e., nanotwists, nanoribbons and nanofibers) from the azobenzene-glycopeptides (AGPs) under the same conditions when the position of the Ala residue was switched. Experimental methods including transmission electron microscopy (TEM), atomic force microscopy (AFM), X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy and circular dichroism (CD) spectroscopy were used to characterize the structural details of glycopeptide mimetic assemblies. Martini coarse-grained molecular dynamics (MD) simulations confirmed such structural observations and investigated the differences in assembly mechanisms. Furthermore, the glycopeptide mimetic assemblies showed a reversible disassembly–assembly process in response to temperature, light or host–guest chemistry, and can be used as switchable antibiofilm nanoagents.


 Please wait while we load your content...
Please wait while we load your content...