Nanolayered cobalt–molybdenum sulphides (Co–Mo–S) catalyse borrowing hydrogen C–S bond formation reactions of thiols or H2S with alcohols†
Abstract
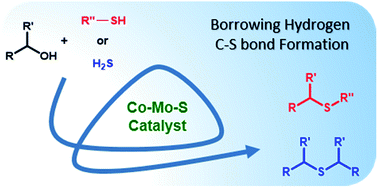
Nanolayered cobalt–molybdenum sulphide (Co–Mo–S) materials have been established as excellent catalysts for C–S bond construction. These catalysts allow for the preparation of a broad range of thioethers in good to excellent yields from structurally diverse thiols and readily available primary as well as secondary alcohols. Chemoselectivity in the presence of sensitive groups such as double bonds, nitriles, carboxylic esters and halogens has been demonstrated. It is also shown that the reaction takes place through a hydrogen-autotransfer (borrowing hydrogen) mechanism that involves Co–Mo–S-mediated dehydrogenation and hydrogenation reactions. A novel catalytic protocol based on the thioetherification of alcohols with hydrogen sulphide (H2S) to furnish symmetrical thioethers has also been developed using these earth-abundant metal-based sulphide catalysts.


 Please wait while we load your content...
Please wait while we load your content...