Catalytic asymmetric allylation of aldehydes with alkenes through allylic C(sp3)–H functionalization mediated by organophotoredox and chiral chromium hybrid catalysis†
Abstract
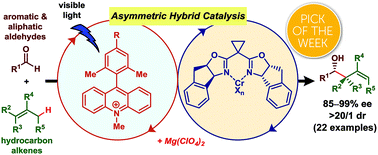
We describe a hybrid system that realizes cooperativity between an organophotoredox acridinium catalyst and a chiral chromium complex catalyst, thereby enabling unprecedented exploitation of unactivated hydrocarbon alkenes as precursors to chiral allylchromium nucleophiles for asymmetric allylation of aldehydes. The reaction proceeds under visible light irradiation at room temperature, affording the corresponding homoallylic alcohols with a diastereomeric ratio >20/1 and up to 99% ee. The addition of Mg(ClO4)2 markedly enhanced both the reactivity and enantioselectivity.
- This article is part of the themed collections: Most popular 2019-2020 catalysis articles, Most popular 2018-2019 catalysis articles, 2019 Chemical Science HOT Article Collection, Editor's choice - Paolo Melchiorre and 2019 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...