Total synthesis of micrococcin P1 and thiocillin I enabled by Mo(vi) catalyst†
Abstract
Thiopeptides are a class of potent antibiotics with promising therapeutic potential. We developed a novel Mo(VI)-oxide/picolinic acid catalyst for the cyclodehydration of cysteine peptides to form thiazoline heterocycles. With this powerful tool in hand, we completed the total syntheses of two representative thiopeptide antibiotics: micrococcin P1 and thiocillin I. These two concise syntheses (15 steps, longest linear sequence) feature a C–H activation strategy to install the trisubstituted pyridine core and thiazole groups. The synthetic material displays promising antimicrobial properties measured against a series of Gram-positive bacteria.
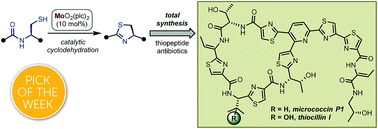
- This article is part of the themed collections: 2019 Chemical Science HOT Article Collection and 2018 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...