Under-cover stabilization and reactivity of a dense carbon monoxide layer on Pt(111)†
Abstract
The space between a metal surface and a two-dimensional cover can be regarded as a nanoreactor, where confined molecule adsorption and surface reactions may occur. In this work, we report CO intercalation and reactivity between a graphene-hexagonal boron nitride (h-BNG) heterostructure and Pt(111). By employing high resolution X-ray photoemission spectroscopy (XPS) we demonstrate the molecular intercalation of the full h-BNG overlayer and stabilization of a dense 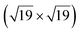 R23.4°–13CO layer on Pt(111) under ultra-high vacuum at room temperature. We provide experimental evidence of a weakened CO–metal bond due to the confinement effects of the 2D cover. Temperature-programmed XPS results reveal that CO desorption is kinetically delayed and occurs at a higher temperature than on bare Pt(111). Moreover, CO partially reacts with the h-BNG layer to form boron-oxide species, which affect repeated CO intercalation. Finally, we found that the properties of the system towards interaction with CO can be considerably recovered using high temperature treatment.
R23.4°–13CO layer on Pt(111) under ultra-high vacuum at room temperature. We provide experimental evidence of a weakened CO–metal bond due to the confinement effects of the 2D cover. Temperature-programmed XPS results reveal that CO desorption is kinetically delayed and occurs at a higher temperature than on bare Pt(111). Moreover, CO partially reacts with the h-BNG layer to form boron-oxide species, which affect repeated CO intercalation. Finally, we found that the properties of the system towards interaction with CO can be considerably recovered using high temperature treatment.


 Please wait while we load your content...
Please wait while we load your content...