A Golgi-targeting fluorescent probe for labile Fe(ii) to reveal an abnormal cellular iron distribution induced by dysfunction of VPS35†
Abstract
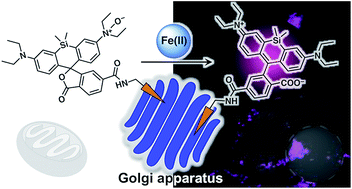
Iron is involved in numerous physiologically essential processes in our body. However, excessive iron is a pathogenic factor in neurodegenerative diseases, causing aberrant oxidative stress. Divalent metal transporter 1 (DMT1) acts as a primary transporter of Fe(II) ions. The intracellular delivery of DMT1 toward the cellular membrane via the trans-Golgi network during the endocytotic process is partially regulated by a retromer-mediated protein-sorting system comprising vacuolar protein-sorting proteins (VPSs). Thus, together with DMT1, the Golgi-apparatus acts as a hub organelle in the delivery system for intracellular Fe(II) ions. Dysfunction of the VPS-relevant protein sorting system can induce the abnormal delivery of DMT1 toward lysosomes concomitantly with Fe(II) ions. To explore this issue, we developed a fluorescent probe, Gol-SiRhoNox, for the Golgi-specific detection of Fe(II) ions by integrating our original N-oxide-based Fe(II)-specific chemical switch, a new Golgi-localizable chemical motif, and polarity-sensitive fluorogenic scaffold. Our synchronous imaging study using Gol-SiRhoNox and LysoRhoNox, a previously developed fluorescent probe for lysosomal Fe(II), revealed that the intracellular distribution balance of Fe(II) ions between the Golgi apparatus and lysosomes is normally Golgi-dominant, whereas the lysosome-specific elevation of Fe(II) ions was observed in cells with induced dysfunction of VPS35, a member of the retromer complex. Treatment of cells with dysfunctional VPS35 with R55, a molecular chaperone, resulted in the restoration of the subcellular distribution of Fe(II) ions to the Golgi-dominant state. These results indicate that the impairment of the DMT1 traffic machinery affects subcellular iron homeostasis, promoting Fe(II) leakage at the Golgi and lysosomal accumulation of Fe(II) through missorting of DMT1.


 Please wait while we load your content...
Please wait while we load your content...