Photoredox-mediated remote C(sp3)–H heteroarylation of free alcohols†
Abstract
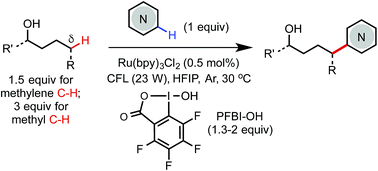
We report an efficient and economical method for remote δ C(sp3)–H heteroarylation of free aliphatic alcohols using a hypervalent iodine PFBI-OH oxidant under photoredox catalysis. The reaction sequence involves in situ alcoholysis of PFBI-OH with alcohol, generation of an alkoxy radical intermediate by SET reduction, 1,5-HAT, and Minisci-type C–C bond formation. This method uses a slight excess of alcohols, can facilitate reaction at δ methyl and methylene positions, and has been successfully applied to modification of complex drug molecules.
- This article is part of the themed collections: Celebrating 100 Years of Chemistry at Nankai University, Most popular 2019-2020 organic chemistry articles, Most popular 2018-2019 organic chemistry articles, Editor's choice - Paolo Melchiorre and 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...