Counterion influence on dynamic spin properties in a V(iv) complex†
Abstract
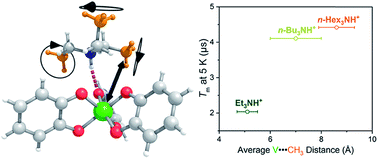
Using transition metal ions for spin-based applications, such as electron paramagnetic resonance imaging (EPRI) or quantum computation, requires a clear understanding of how local chemistry influences spin properties. Herein we report a series of four ionic complexes to provide the first systematic study of one aspect of local chemistry on the V(IV) spin – the counterion. To do so, the four complexes (Et3NH)2[V(C6H4O2)3] (1), (n-Bu3NH)2[V(C6H4O2)3] (2), (n-Hex3NH)2[V(C6H4O2)3] (3), and (n-Oct3NH)2[V(C6H4O2)3] (4) were probed by EPR spectroscopy in solid state and solution. Room temperature, solution X-band (ca. 9.8 GHz) continuous-wave electron paramagnetic resonance (CW-EPR) spectroscopy revealed an increasing linewidth with larger cations, likely a counterion-controlled tumbling in solution via ion pairing. In the solid state, variable-temperature (5–180 K) X-band (ca. 9.4 GHz) pulsed EPR studies of 1–4 in o-terphenyl glass demonstrated no effect on spin–lattice relaxation times (T1), indicating little role for the counterion on this parameter. However, the phase memory time (Tm) of 1 below 100 K is markedly smaller than those of 2–4. This result is counterintuitive, as 2–4 are relatively richer in 1H nuclear spin, hence, expected to have shorter Tm. Thus, these data suggest an important role for counterion methyl groups on Tm, and moreover provide the first instance of a lengthening Tm with increasing nuclear spin quantity on a molecule.


 Please wait while we load your content...
Please wait while we load your content...