A copper-catalyzed double coupling enables a 3-step synthesis of the quassinoid core architecture†
Abstract
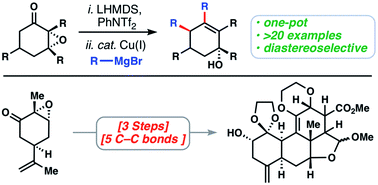
The quassinoids are a fascinating class of degraded triterpene natural products which possess, among other attributes, potent anti-cancer activity. Their complex polycyclic ring systems also serve as inspiration for the development of new chemical methods and strategies – especially those pertaining to C–C bond formation. Herein we disclose a novel tandem cross coupling/SN2′ reaction of vicinal epoxy vinyl triflates with simple Grignard reagents catalyzed by Cu(I). Using this transformation, the polycyclic core architecture of the quassinoids can be generated in only three linear steps from carvone epoxide, forming five carbon–carbon bonds in the process.


 Please wait while we load your content...
Please wait while we load your content...