Analysis of the autothermal operability of the Sabatier reaction in a heat-recirculating microreactor using CFD†
Abstract
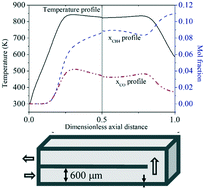
The Sabatier reaction, which is the catalytic reduction of CO2 to methane, is a reversible and exothermic reaction. The reaction provides a classical trade-off, with low temperature thermodynamically favored, and high temperature required for the occurrence of the reaction. It is also accompanied by side reactions that produce CO. In this work, the autothermal operability of the Sabatier reaction is analyzed in a thermally integrated U-bend microreactor using computational fluid dynamics (CFD) simulations. Internal recirculation of heat from the hot product stream to the cold inlet stream results in a favorable temperature profile. Results show that there is sufficient preheating near the inlet to overcome the kinetic barrier so that the reaction lights-off; and simultaneous cooling of the product stream near the outlet, which thermodynamically favors higher conversion to methane. This preheating through internal heat recirculation enables the U-bend reactor to be used even with feed at ambient temperature, a unique feature not present in a non-heat-recirculating straight-channel reactor with otherwise similar properties. Comparisons with other modes of operation, and the effects of the operating conditions, namely inlet velocity, temperature and composition, are also presented.


 Please wait while we load your content...
Please wait while we load your content...