Selective recognition of human telomeric G-quadruplex with designed peptide via hydrogen bonding followed by base stacking interactions†
Abstract
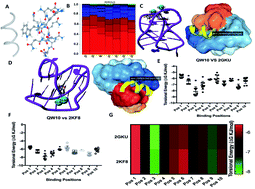
We described a novel synthetic peptide in which a glutamine residue binds through hydrogen bonding to a guanine-base and a trytophan residue intercalates with K+ resulting in stabilization of a human telomeric G-quadruplex with high selectivity over its complementary c-rich strand and a double-stranded DNA and its complementary C-rich strand. This peptide offers great potential for cancer treatment by inhibiting the telomere extension by telomerase.


 Please wait while we load your content...
Please wait while we load your content...