Synthesis and biological evaluation of geldanamycin–ferulic acid conjugate as a potent Hsp90 inhibitor†
Abstract
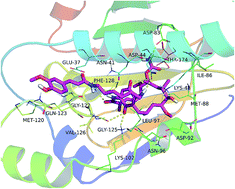
A novel geldanamycin–ferulic acid conjugate LZY228 was prepared and evaluated for anti-proliferation activity on human cancer cell line MDA-MB-231. Compound LZY228 exhibited potent cytotoxicity with IC50 value of 0.27 μM, which was more potent than 17-AAG. Hepatotoxicity test in mice demonstrated that the levels of both AST and ALT of LZY228-treated group were lower than that of GA-treated group, indicating that LZY228 was a promising antitumor candidate. In addition, excellent in vivo antitumor potency of LZY228 was observed in MDA-MB-231 xenograft model, which was superior to reference drug 17-AAG. Docking and MD refinement of the Hsp90-LZY228 complex give us an explanation of theoretical binding model of 17-ferulamido-17-demethoxygeldanamycins at molecular level.


 Please wait while we load your content...
Please wait while we load your content...