Removal of anthracene in water by MIL-88(Fe), NH2-MIL-88(Fe), and mixed-MIL-88(Fe) metal–organic frameworks†
Abstract
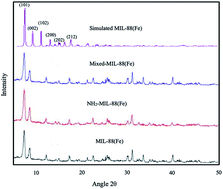
Three adsorbents based on the metal–organic frameworks (MOFs), viz.; MIL-88(Fe), NH2-MIL-88(Fe), and mixed-MIL-88(Fe) were synthesized using a microwave-assisted solvothermal technique. The as-synthesized MOFs were characterized by X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET), field emission scanning microscopy (FESEM), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), and Fourier transform infrared spectroscopy (FTIR). The MOFs were shown to possess highly crystalline and porous structures with specific surface areas of 1240, 941, and 1025 m2 g−1 and pore volumes of 0.7, 0.6 and 0.6 m3 g−1 for MIL-88(Fe), NH2-MIL-88(Fe) and mixed-MIL-88(Fe), respectively. Faster removal of a model polycyclic aromatic hydrocarbon, anthracene (ANT) within 25 minutes, was achieved when these MOFs were used as adsorbents in water. The removal efficiency was 98.3, 92.4 and 95.8% for MIL-88(Fe), NH2-MIL-88(Fe) and mixed-MIL-88(Fe), respectively. The kinetics and isotherms of the process were best statistically described by pseudo-second-order and Langmuir models, respectively, while the thermodynamic studies revealed the exothermic and spontaneous nature of the process. Docking simulations were found to be consistent with the experimental results with MIL-88(Fe) showing the best binding capacity with the ANT molecule.


 Please wait while we load your content...
Please wait while we load your content...