Syntheses, characterization and properties of three coordination polymers with interpenetrating structures comprising 4,4′-(1H-1,2,4-triazol-1-yl)methylene-bis(benzonic acid)†
Abstract
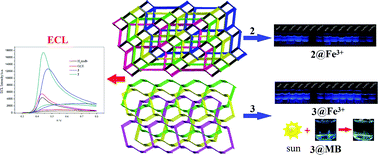
Three new coordination polymers (CPs), {[Pb(tmdb)](H2O)}n (1), {[Zn(tmdb)(bimb)0.5]}n (2) and {[Zn3(tmdb)3(bpmb)1.5](H2O)6}n (3) (H2tmdb = 4,4′-(1H-1,2,4-triazol-1-yl)methylene-bis(benzonic acid), where bpmb = 1,4-bis(pyridin-4-ylmethoxy)benzene and bimb = 1,4-bis(imidazoly-1-yl)benzene), have been solvothermally or hydrothermally synthesized. Compound 1 is a 2D network with the point symbol (4·6·8)(4·62) and compound 2 is a 4-fold interpenetrating 3D network with spiral chains. The topological type of 2 is dmc (topos&RCSR.ttd) with the point symbol (4·82)(4·85). Compound 3 is a 3-fold interpenetrating 3D network with the point symbol (63)2(8·65)2(10·62)(8·10·64). The electrochemiluminescence (ECL) behaviors of 2 and 3 were studied. The applications of CP 2 and 3 in detecting ions were explored, and the results show that they can be used as fluorescent probes to selectively detect and identify Fe3+ ions in water. In addition, the applications of CP 2 and 3 in the adsorption and separation of dyes were researched. Furthermore, the gas adsorption of 3 was studied.


 Please wait while we load your content...
Please wait while we load your content...